|
|
|
|||||||||||||||||||||||
|
2 Methods of Plant Breeding2.1 Conventional MethodsPlant breeding is defined as identifying and selecting desirable traits in plants and combining these into one individual plant. Since 1900, Mendel's laws of genetics provided the scientific basis for plant breeding. As all traits of a plant are controlled by genes located on chromosomes, conventional plant breeding can be considered as the manipulation of the combination of chromosomes. In general, there are three main procedures to manipulate plant chromosome combination. First, plants of a given population which show desired traits can be selected and used for further breeding and cultivation, a process called (pure line-) selection. Second, desired traits found in different plant lines can be combined together to obtain plants which exhibit both traits simultaneously, a method termed hybridization. Heterosis, a phenomenon of increased vigor, is obtained by hybridization of inbred lines. Third, polyploidy (increased number of chromosome sets) can contribute to crop improvement. Finally, new genetic variability can be introduced through spontaneous or artificially induced mutations.[2]
Success in using conventional plant breeding principles and agricultural techniques reached its peak when high-yielding wheat and rice lines were cultivated in the 1960s. The doubling and tripling of productivity of these important crops in Asia signaled a agricultural revolution in the developing countries. This breakthrough in food production was termed the "Green Revolution" to describe the social, economic, and nutritional impact of the new high-yielding wheat and rice strains. Norman Borlaug was awarded the Noble Price in 1970 for his contribution in breeding new high-yielding strains of cereals. However, these strains were highly dependent on fertilizers, irrigation and agrochemicals and required energy-intensive investments. This led to degradation and loss of soils and other severe environmental problems all over the world. For these reasons, the "Green Revolution" has been both praised and damned.[2] 2.2 Biotechnological MethodsBiotechnology is the discipline which deals with the use of living organisms or their products. In this wide sense, also traditional agriculture may be seen as a form of biotechnology. The European Federation of Biotechnology defines biotechnology as "the integrated use of biochemistry, microbiology and engineering sciences in order to achieve technological (industrial) application of the capability of microorganisms, cultured tissue cells and parts thereof". In recent years, biotechnology has developed rapidly as a practical means for accelerating success in plant breeding and improving economically important crops. The most important methods used to achieve these goals are described below. The techniques of genetic engineering, which are a part of biotechnology, will be discussed in more detail in the next chapter.
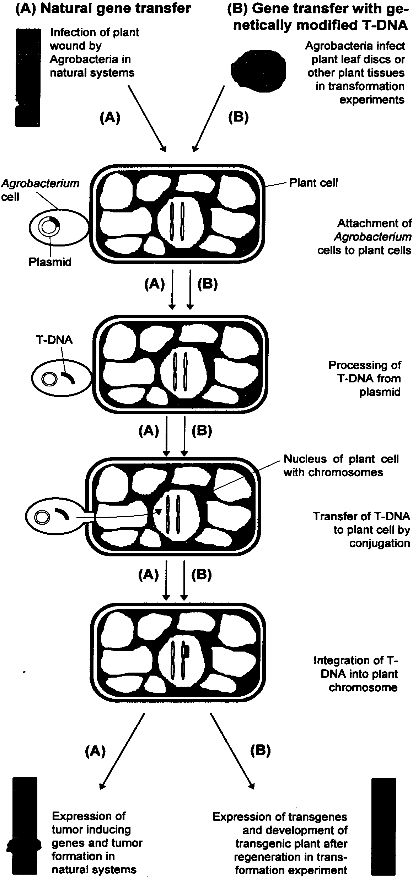
2.3 Genetic EngineeringGenetic engineering is a term used for the directed manipulation of genes, i. e. the transfer of genes between organisms or changes in the sequence of a gene. Closely related to this field are methods which use genes or specific sequences for the identification of traits and other analytical purposes. In plant breeding, the most important and already widely used method of this kind is Restriction Fragment Length Polymorphism (RFLP). The basics of this technique are described below. The principles of plant genetic engineering will be described in the next chapter. For those who are not familiar with the principles of genetics, the glossary at the end of this report explains the most important terms. 2.3.1 Restriction Fragment Length Polymorphism (RFLP)The techniques of traditional breeding are very time-consuming. By making crosses, also a large number of undesired genes is introduced into the genome of the plant. The undesired genes have to be "sorted out" by back-crossing. The use of Restriction Fragment Length Polymorphism greatly facilitates conventional plant breeding, because one can progress through a breeding program much faster, with smaller populations and without relying entirely on testing for the desired phenotype. RFLP makes use of restriction endonucleases. These are enzymes which recognize and cut specific nucleotide sequences in DNA. For example, the sequence GAATTC is cut by the endonuclease EcoRl. After treatment of a plant genome which restriction endonucleases, the plant DNA is cut into pieces of different length, depending on the number of recognition sites on the DNA. These fragments can be separated according to their size by using gel electrophoresis and are made visible as bands on the gel by hybridizing the plant DNA fragments with radiolabeled or fluorescent DNA probes. As two genomes are not identical even within a given species due to mutations, the number of restriction sites and therefore the length and numbers of DNA fragments differ, resulting in a different banding pattern on the electrophoresis gel. This variability has been termed restriction fragment length polymorphism (RFLP). The closer two organisms are related, the more the pattern of bands overlap. If a restriction site lies close to or even within an important gene, the existence of a particular band correlates with the particular trait of a plant, e.g. disease resistance. By looking at the banding pattern, breeders can identify individuals which have inherited resistance genes, and resistant plants can be selected for further breeding. The use of this technique will not only accelerate progress in plant breeding considerably, but will also facilitate the identification of resistance genes, thereby opening new possibilities in plant breeding (compare also chapter 3.1.3.1).[4] 2.3.2 Gene TransferIn conventional breeding, the pool of available genes and the traits they code for is limited due to sexual incompatibility to other lines of the crop in question and to their wild relatives. This restriction can be overcome by using the methods of genetic engineering, which in principle allow introducing valuable traits coded for by specific genes of any organism (other plants, bacteria, fungi, animals, viruses) into the genome of any plant. The first gene transfer experiments with plants took place in the early 1980s. Normally, transgenes are inserted into the nuclear genome of a plant cell. Recently it has become possible to introduce genes into the genome of chloroplasts and other plastids (small organelles of plant cells which possess a separate genome). The advantages of this technique are discussed in chapter 3.1.1.1. Transgenic plants have been obtained using Agrobacterium-mediated DNA-transfer and direct DNA-transfer, the latter including methods such as particle bombardment, electroporation and polyethylenglycol permeabilisation. The majority of plants have been transformed using Agrobacterium mediated transformation.[5] 2.3.2.1 Agrobacterium-mediated Gene TransferThe Agrobacterium-mediated technique involves the natural gene transfer system resident in the bacterial plant pathogens of the genus Agrobacterium. In nature, Agrobacterium tumefaciens and Agrobacterium rhizogenes are the causative agents of the crown gall and the hairy root diseases, respectively. The utility of Agrobacterium as a gene transfer system was first recognized when it was demonstrated that these plant diseases were actually produced as a result of the transfer and integration of genes from the bacteria into the genome of the plant. Both Agrobacterium species carry a large plasmid (small circular DNA molecule) called Ti in A. tumefaciens and Ri in A. rhizogenes. A segment of this plasmid, designated T-(for transfer) DNA, is transmitted by this organism into individual plant cells, usually within wounded tissue (see figure 2.1). The T-DNA segment penetrates the plant cell nucleus and integrates randomly into the genome where it is stably incorporated and inherited like any other plant gene in a predictable, dominant Mendelian fashion. Expression of the natural genes on the T-DNA results in the synthesis of gene products that direct the observed morphological changes, i. e. tumor or hairy root formation. In genetic engineering, the tumor inducing genes within the T-DNA which cause the plant disease are removed and replaced by foreign genes. These genes are then stably integrated into the genome of the plant after infection with the altered strain of Agrobacterium, just like the natural T-DNA. Because all tumor-inducing genes are removed, the gene transfer does not induce any disease symptoms. The most important steps in Agrobacterium-mediated gene transfer are shown in figure 2.1.This reliable method of gene transfer is well suited for plants which are susceptible to infection by Agrobacterium. Unfortunately, many species, especially economically important legumes and monocotyledons such as cereals, do not respond positively to Agrobacterium-mediated transformation. For these plants, the following methods of direct DNA uptake must be applied.[5] 2.3.2.2 Particle BombardmentThis method, also referred to as biolistic transformation (from biological ballistics) involves coating biologically active DNA onto small tungsten or gold particles (1-5 ?m in diameter) and accelerating them into plant tissue at high velocity. The particles penetrate the plant cell wall and lodge themselves within the cell where the DNA is liberated resulting in transformation of the individual plant cell in an explant. This technique is generally less efficient than Agrobacterium-mediated transformation, but has nevertheless been particularly useful in several plant species, most notably in cereal crops. The introduction of DNA into organized, morphogenic tissues such as seeds, embryos or meristems has enabled the successful transformation and regeneration of rice, wheat, soybean and maize, thus demonstrating the enormous potential of this method .[5] 2.3.2.3 Electroporation and Direct DNA Entry into ProtoplastsElectroporation is a process whereby very short pulses of electricity are used to reversibly permeabilize lipid bilayers of plant cell membranes. The electrical discharge enables the diffusion of macromolecules such as DNA through an otherwise impermable plasma membrane. Because the plant cell wall will not allow the efficient diffusion of many transgene constructs, protoplasts (cells without cell walls) must be prepared. This requirement presents a major obstacle for many applications as protocols making possible the regeneration of protoplasts into complete plants do not exist for many species.
DNA uptake by plant protoplasts can also be stimulated by phosphate or calcium/polyethylene glycol (PEG) coprecipitation. However, these methods all suffer from the drawback that they use protoplasts as the recipient host which often cannot be regenerated into whole plants. [5] 2.3.3 Transgene ExpressionIn most cases, the introduction of a gene into the plant genome will only have an effect on the plant if the transgene is expressed, i. e. transcribed into mRNA and translated into a protein. A promoter is a sequence of nucleic acids where the RNA polymerase (a complex enzyme synthesizing the mRNA transcript) attaches to the DNA template. The nature of the promoter defines (together with other expression-regulating elements), under which conditions and with which intensity a gene will be transcribed. The promoter of the 35S gene of cauliflower mosaic virus is used very frequently in plant genetic engineering. This promoter confers high-level expression of exogenous genes in most cell types from virtually all species tested. As it is often advantageous to express a transgene only in certain tissues or quantities or at certain times, a number of other promoters are available, e.g. promoters inducing gene expression after wounding or during fruit ripening only. Methods of gene transfer currently employed result in the random integration of foreign DNA throughout the genome of recipient cells. The site of insertion may have a strong influence on the expression levels of the exogenous gene, resulting in different expression levels of an introduced gene, even if the same promoter/gene construct was used. The exact mechanism of this phenomenon are not yet fully understood. [5] 2.3.4 Selection and Plant RegenerationIn a transformation experiment, the proportion of transformed cells is usually small compared to the number of cells which remain unaltered. In order to select only cells which have actually incorporated the new genes, the genes coding for the desired trait are fused to a gene which allows selection of transformed cells, so-called marker genes. The expression of the marker gene enables the transgenic cells to grow in presence of a selective agent, usually an antibiotic or a herbicide, while cells without the marker gene die. One of the most commonly used marker genes is the bacterial aminoglycoside-3' phosphotransferase gene (APH(3')II), also referred to as neomycin phosphotransferase 11 (NPTII). This gene codes for an enzyme which inactivates the antibiotics kanamycin, neomycin and G418 through phosphorylation. In addition to NPTII, a number of other antibiotic resistance genes have been used as selective markers, e.g. hygromycin phosphotransferase gene conferring resistance to hygromycin. Another group of selective markers are herbicide tolerance genes. Herbicide tolerance has been obtained through the incorporation and expression of a gene which either detoxifies the herbicide in a similar manner as the antibiotic resistance gene products or a gene that expresses a product which acts like the herbicide target but is not affected by the herbicide. Herbicide tolerance may not only serve as a trait useful for selection in the development of transgenic plants, but also has some commercial interest. Herbicide tolerance transgenic plants are therefore among the first crops approaching market introduction. Transformation of plant protoplasts, cells and tissues is usually only useful if the they can be regenerated into whole plants. The rates of regeneration vary greatly not only among different species, but also between cultivars of the same species. As mentioned in capter 2.2, in many cases regeneration of whole plants from cells is not possible or very difficult. Besides the ability to introduce a gene into the genome of a plant species, regeneration of intact, fertile plants out of transformed cells or tissues is the most limiting step in developing transgenic plants.[5]
|
||||||||||||||||||||||||
|
|




 Produkten.
Produkten.